Results from the Oxford-managed PRINCIPLE trial of ivermectin in out-patient Covid-19 were published in the Journal of Infection on 29 February 2024, over a year and half after the trial’s closure in July 2022, and over 2 years since the originally planned termination. The unexpected pause in January 2022 (said to be due to supply problems, denied by the supplier) remains unexplained. PRINCIPLE is funded by the NIHR, i.e. by the British taxpayer.
The trial design was criticised by FLCCC and BiRD International on 1 July 2021, because of enrolment up to 14 days of symptoms. The comprehensive summary of clinical evidence at c19ivm.org thus categorises this as a “Late Treatment” RCT. The protocol treatment, with weak dosing, short duration, fasting administration minimising serum concentrations, confined to a monotherapy with disregard of ubiquitous adjuncts, and of the concurrent corticosteroids indicated at later disease stages, did not correspond to any treatments adopted by clinicians using ivermectin with success. Overall the majority of patients were low-risk, after enrolment criteria were changed in July 2021 to accommodate all adults.
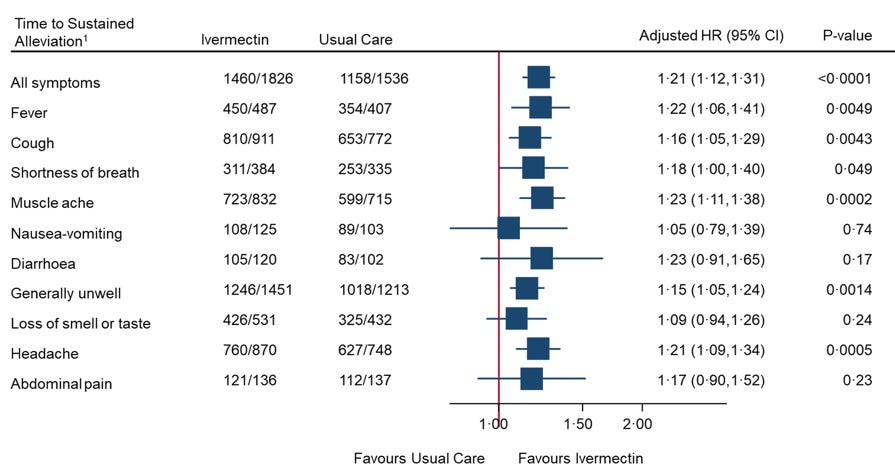
In spite of the low-risk population, under-dosing, and late treatment, statistically significant improvements in times to recovery on various metrics are found, Fig. S6(b) opposite, though not graphed in the main paper. The authors’ summary rejects these as not clinically significant, based on an arbitrary, pre-defined, metric: – “Ivermectin for COVID-19 is unlikely to provide clinically meaningful improvement in recovery, hospital admissions, or longer-term outcomes. Further trials of ivermectin for SARSCov-2 infection in vaccinated community populations appear unwarranted.” This does not follow from the evidence. A rational response to statistically significant improvement in recovery time, demonstrating a positive quantified effect, would be to seek to optimise the protocol. Also the alleviation of Post-Acute Sequelae of SARS-Cov-2 (PASC) i.e. “Long Covid”, as detailed here from data in the Supplementaries, is clear, and at variance with the negative conclusion on “longer term outcomes”.
BiRD International and the World Council for Health agree however that further trials of ivermectin alone are unwarranted, the weight of evidence summarised at covid19.org being so overwhelming that the remaining issues concern dosage, adjuncts, and mechanism, rather than further empirical evidence of clinical effect. The paper’s opening statement “The evidence … is contested” does not survive scrutiny of all available evidence, even after this report. Multiple critiques of design and implementation remain unanswered, data sharing is only offered subject to contracts, and an undeclared conflict of interest is Prof Chris Butler’s parallel PANORAMIC trial of molnupiravir, a proprietary drug.
We find the results unsurprising in view of the poor trial design; they do little or nothing to refute the accumulated worldwide evidence on the efficacy of ivermectin in Covid-19.